HYDROGEN is the lightest and most abundant element in the universe as well as the source of all energy. Deep within the sun and stars, nuclear fusion converts hydrogen into helium. The energy that is released when four hydrogen atoms become a helium atom is the energy which fuels all life. Evidence of the incredible amount of energy contained within a hydrogen atom is the thermonuclear or hydrogen bomb, which exploits nuclear fusion to release its destructive power.
In our natural environment, hydrogen exists primarily in combination with other elements. In order for hydrogen to be useful as a fuel, it must exist as H2 or “free hydrogen.” H2 must therefore be produced, unlike fossil fuels such as natural gas, coal and oil which can be directly mined or extracted. In this sense, hydrogen is a secondary source of energy, analogous to electricity. The energy used to produce H2 is stored, with some losses, within the H2 molecule. This energy can then be kept in storage, used on-site, or transported to a remote location for energy conversion. The fact that hydrogen must be produced is a major consideration when examining its effectiveness as an energy carrier, and is the biggest stumbling-block to widespread use in commercial applications.
Free hydrogen exists at normal atmospheric conditions as an odorless, colorless gas. It is stable and will co-exist harmlessly with free oxygen (O2) until an input of energy drives the exothermic (heat-releasing) reaction which forms water. This reaction from a higher energy state to a lower one generates a positive output of energy. For over a century it has been predicted that a system will be developed in which hydrogen, extracted from pure water using energy derived from the sun, is used as a fuel or as an “energy-carrier,” and will serve to provide the demands for all of society’s power requirements. The beauty of the system being that solar energy and water, the sources, are practically limitless and that the resulting energy conversion is relatively pollution-free with the only waste product being pure water. A seemingly perfect cycle, beginning and ending with energy and water.
In 1870, Jules Verne predicted with impressive foresight the use of hydrogen fuel in his Sci-Fi classic Mysterious Island. Verne describes a process whereby, “…water will one day be employed as fuel, that hydrogen and oxygen which constitute it, used singly or together, will furnish an inexhaustible source of heat and light, of an intensity of which coal is not capable. …Water will be the coal of our future.” Where is this technology that has had 100 years to come to fruition? An examination of the history of hydrogen research as well as a look at today’s research and development helps to provide some answers.
History
Probably the first recorded event of the production of hydrogen in the laboratory is contained within 15th century alchemical texts. The alchemists dealt extensively with the transmutation of metals, a procedure which required the dissolution of metals in salts or acids. At the time, the existence of the element hydrogen was unknown to the alchemists, although they were aware of the presence of something different in these metal/acid reactions. Theophrastus Bombastus “Paracelsus” (1493-1541) was purported to have said, when he dissolved iron in spirit of vitriol, “Air arises and breaks forth like the wind.” He was most probably referring to the production of hydrogen. Still, little was understood of the gas’ properties. Its burnability was not noted until the 17th century by Turquet de Mayerne. [4]
It was the common belief in 17th century Europe that air itself was a basic element. Some perceptive individuals suspected that there existed a property of air which was required for the combustion and the sustenance of life. Some important figures involved in this quest were the Dutch physician Herman Boerhaave (1668-1738), the English scientist Robert Boyle (1627-1691) (who developed Boyle’s Law) and the English physician John Mayow (1645-1679) all of which were outspoken in their belief in a “life-giving” substance within air.
It was also believed that there existed a substance called phlogiston which imparts burnability in matter and that combustion was the release of phlogiston. This theory was first published in 1697 by the German scientist Georg Ernst Stahl (1660-1734). Henry Cavendish (1731-1810), believing in the existence of phlogiston, attempted to describe some of its properties. He succeeded in isolating carbon dioxide (CO2) and hydrogen gas (H2) and dubbed them “fixed air” and “flammable air” respectively. He was able to obtain precise measures of hydrogen’s specific weight and density, although he thought he was studying [47] a pure state of phlogiston. Cavendish also discovered that igniting a mixture of flammable air and oxygen (air) produced water. These were to be pivotal investigations into the properties of hydrogen.
The French chemist Antoine Laurent Lavoisier (1743-1794) continued the study of flammable air, repeating Cavendish’s experiments, and eventually produced hydrogen and oxygen in the laboratory via the dissolution of metals in acid. He was also able to split water molecules using a heated copper tube. In another experiment he combined hydrogen and oxygen and produced water. These experiments, in 1785, were to prove definitively that H2 and O2 are the basic constituents of water. It was his important publication, The Method of Chemical Nomenclature, in which Lavoisier named the “flammable air” hydrogen, and the “life-sustaining air” oxygen. Lavoisier was eventually executed after the French Revolution in 1794 because of his associations with the pre-Revolution French government, a loss heavily mourned by the international scientific community of the day.
The discovery that “flammable air” was fourteen times lighter than air led to the use of hydrogen as a bouyant in aeronautical balloons. A French physicist Jacques Alexandre Cesar Charles (1746-1823) was the first to use hydrogen in a balloon known as a “Charliere,” in which he was able to fly to an altitude of 3km in 1783 (use of hydrogen continued on into the 20th century, eventually being replaced by helium due to its inert properties, thus reducing chance of explosion).
Soon after Alessandro Volta built his first electric cell near the turn of the century, two English scientists, William Nicholson and Sir Anthony Carlisle, discovered that by passing an electric current through water, hydrogen and oxygen could be produced. This process, called electrolysis, was to become an important method for the production of hydrogen. In 1839, Sir William Groves was able to reverse the process, combining hydrogen and oxygen with platinum electrodes and a sulfuric acid (H2SO4) electrolyte to produce electricity and water, inventing the first fuel cell.
Early in the 19th century, the Reverend William Cecil presented a paper to the Cambridge Philosophical Society entitled, “On the Application of Hydrogen Gas to Produce Moving Power in Machinery.” Basically, the paper described a hydrogen-powered engine in which hydrogen and oxygen were combined and ignited. The ensuing vacuum generated a moving force via the air that rushed in to fill the void. Although there is no record that the engine was actually built, Cecil’s proposal pioneered the study of hydrogen’s use as a fuel.
On into the 20th century, hydrogen’s development as a fuel source had achieved little progress until the Scottish geneticist J.B.S. Haldane presented a paper to Cambridge University in which he proposed that Britain could meet it’s increasing demand for energy by using wind energy to electrolyze water into hydrogen and oxygen. The gases, first liquefied, can be stored in underground reservoirs until needed. They can then be recombined in combustion motors or “oxidation cells.” This paper, presented in 1923, offered a glimpse into the potential of a solar-hydrogen fuel system. At a time when the use of fossil fuels, especially coal, was prevalent, Haldane emphasized the scarcity of fossil fuels and the eventual necessity of an alternative source of energy.
Haldane also pointed out that liquid hydrogen has three times as much heat per pound of hydrocarbon fuel. This is an important factor when developing hydrogen fuel for use in air and space travel, where weight is of a prime design criteria. However, the lightness of hydrogen allows for only about one-third of the energy per unit volume. An aircraft using hydrogen will be able to fly higher and farther due to the lighter fuel load than an aircraft using the energy-equivalent amount of jet fuel. [4][13] The tanks, however, must be much larger and take up to one-third of the fuselage in current designs for commercial aircraft. [4]
In the 1930′s, interest in hydrogen as a fuel reached a new height. In Germany, two men were extremely influential in hydrogen research; Franz Lawaczeck and Rudolph Erren. Lawaczeck, a German turbine designer, was sketching designs for hydrogen powered cars as early as 1919. [4] His work, in collaboration with the German-American J.E. Noeggerath, and the German inventor Hermann Oberth, led to ideas for developments in efficient pressurized electrolyzers, liquid hydrogen use as a rocket fuel, and the transportation of hydrogen in pipelines for use as an energy-carrier.
The most influential pioneer of the 1930s would undoubtedly be Rudolf Erren. An expert in the combustion process, Erren began developing hydrogen engines in the late 1920s. He advanced the concept of injecting hydrogen into the air-fuel mixture of combustion engines, serving to heighten the output of the combustion process. Erren, working in cooperation with the German, Australian, and British governments, converted buses, vans, rail cars, and even submarines to be powered by hydrogen or any combination of hydrogen-fuel mixtures. [4][7] However, a conflict of interests brought about by World War II, made it impossible for Erren to work with both the British and German governments and Erren’s efforts to expand the technology were fruitless. Eventually the government research money was cutoff and Erren’s research fell into a period of disquiet.
Other important developments of the 1930s included the work of the German engineer Hermann Honnef, who designed huge wind-power generators which could theoretically produce up to 100 megawatts of power, stored as hydrogen. Although they never went beyond the drawing board, Honnef’s ideas were predecessors to much of the wind-turbine technology used effectively today.
Hydrogen was also being used to supplement fuel in large dirigibles in both Germany and England. In the 1920s and 1930s, before switching to helium, hydrogen was the primary bouyant used in large passenger balloons. These “Zeppelins” were able to fly to altitudes of 2400 feet at 75 mph. The fuel used to drive the motors was typically a benzol-gasoline mixture. In order to maintain proper bouyancy, the captain was required to blow off hydrogen as fuel was consumed. An innovative solution was to [48] combine the blow-off hydrogen with the main fuel in the internal-combustion engines. In this way they were able to decrease fuel consumption. [4] England also used this strategy in their R101 airships, and were also able to reduce the requirements for hydrocarbon fuel.
Zeppelins are still what people most associate with hydrogen, and the Hindenburg disaster is probably the most well-known event involving hydrogen. The Hindenburg’s association to hydrogen has created a negative public image of the gas, and has perpetuated the myth that hydrogen is extremely dangerous. The Hindenburg was actually designed to use helium as the bouyant. At the time of the ill-fated journey to New Jersey, helium was extremely hard to come by due to U.S. trade embargoes. They used hydrogen, the next best thing, but the ship was not equipped with the necessary safety features required to deal with the flammable gas. The explosion was well covered by the media at the time. Little known is the fact that most of the deaths (there were thirty six casualties) were not attributed to the actual explosion, but occured when many tried to jump to safety and died on landing. It is now established that hydrogen is, in fact, less dangerous than most fuels used today. [10]
In the United States, I.I. Sikorski, who developed the first working helicopter, was looking into the use of hydrogen as an aircraft fuel. In 1938, he presented his ideas to the American Institute of Electrical Engineers, suggesting that the use of liquid hydrogen would permit, “… a great change, particularly with respect to long-range aircraft…” “This would make possible the circumnavigation of the earth along the equator in a nonstop flight without refueling. It would also enable an increase in the performance of nearly every type of aircraft.” His statement would prove prophetic in the years to follow.
The next decade saw little in the way of forward progress in hydrogen research and development, presumably due to the distractions of World War II. Any existing collaborations between German and English scientists disintegrated, and funding went elsewhere. The only significant events were the redistribution of fossil-fuel resources, which led many countries to start looking for sources of domestic energy. This factor contributed directly to the work of J.S. Just in Australia, who found that hydrogen, produced via off-peak electricity, cost roughly the same per mile as gasoline in trucks. [7] Plans were made to develop commercial-sized electrolysis plants, but were scrapped after the Allied victory in 1945 made oil available and cheap once again.
One Australian who continued hydrogen research was R.O. King, who relocated to the University of Toronto in Canada to further the cause. From 1948 to 1955, King led his team of scientists at the University of Toronto to conduct numerous studies into the use of hydrogen as an alternative to gasoline in ordinary internal combustion engines. They were able to show that hydrogen was indeed feasible in this capacity, the primary constraint being that the compression ratio in the engines had to be kept below seven to one. [13] These studies in Toronto showed that combustion engines can be converted to run on hydrogen simply and cheaply.
During the same period, the British scientist Francis T. Bacon began development of the hydrogen-air fuel cell. This fuel cell, called the Bacon Cell, substituted an alkali (potassium hydroxide (KOH)) for the acid as the electrolyte, eliminating the problem of corrosion of the electrodes. The Bacon Cell was used as the model fuel cell which was to become an integral part of NASA’s space program.
In the 1950s, the U.S. Air Force was using hydrogen fuel in experimental high-altitude, long-range reconnaissance aircraft. Based at the NACA Lewis Research Center in Ohio, the Air Force converted a B-57 to run on liquid hydrogen. The pilot had the option to switch from the conventional kerosene fuel source to hydrogen. This was fed under pressure from the wing-tip fuel tank to a heat-exchanger where the cryogenic liquid was heated to a gas and burned normally in one of the two jet engines. Although the program was a success, the use of other fuels proved to be more cost-efficient, and the use of hydrogen as an aircraft fuel was discontinued.
At the same time, Lockheed, in conjunction with Pratt & Whitney, was developing a high-altitude, supersonic spy plane to run on liquid hydrogen fuel. This plane, the CL-400, got as far as wind-tunnel testing before the program was discontinued for technical and logistical reasons. A large amount of drag was introduced due to the larger volume requirements of liquid hydrogen storage. This required more power to keep the plane in flight. One positive outcome was the determination that liquid hydrogen did not require more safety precautions than that which were required for hydrocarbon fuels. This is an important step in helping to dispel the “Hindenburg Myth.”
Lockheed and NASA have since continued development of an advanced supersonic transport (AST) using liquid hydrogen fuel (LiqH2). Studies in the tradeoff of reduced fuel consumption with LiqH2 versus increased drag have shown that the LiqH2 AST would still be 43% more efficient (environmental advantages also play a part in the consideration, with reduced carbon emissions, less noise, and lower NOX emissions). [4] Still, the final considerations are economic, and hydrocarbon fuels are still cheaper than hydrogen, when production, storage, and transportation are accounted for. Until the scarcity of fossil fuels makes their price increase to a higher level, government support of LiqH2 aircraft will be lacking.
In the 1960′s NASA developed the use of the hydrogen fuel cell for use in the Apollo missions to the moon. The fuel cells, utilizing expensive platinum electrodes, provided electrical power on-board, as well as generating drinking water for the crew’s consumption. It proved to be a highly reliable system and is still used today in the Space Shuttle missions.
Also, during the 1960s, an Australian electrochemist John O’M. Bockris, while working as a consultant with General Motors, began advancing the idea of a “hydrogen economy.” In this ambitious energy concept, the cities of the United States could be supplied with energy derived from the sun, and the energy stored using hydrogen. GM studied the use of hydrogen for a time, yet did not pursue the technology to any significant [49] degree. Bockris continued his crusade, and the phrase “hydrogen economy,” which has nothing to do with economics, has become an important concept.
In 1966, 16 year old Roger Billings modified a model-A Ford to run on hydrogen. Billings went on to convert many late model automobiles to run on hydrogen using their internal combustion engines. In 1972, he won the anti-pollution category of the Urban Vehicle Design Competition with a hydrogen-fueled Volkswagen. Billings soon teamed up with other interested parties to form one of the most influential advocates and developers of hydrogen-fueled automobiles, the Billings Energy Corporation of Provo. He has demonstrated the feasibility of hydrogen use in buses and mail trucks. Roger Billings is still in the forefront of hydrogen technology, speaking out and demonstrating the advantages of hydrogen use in transportation and home appliances. Since that time, literally hundreds of automobiles have been converted to run on hydrogen.
The “energy crisis” of 1973 produced a major impetus for a renewed interest in alternative fuel sources. The OPEC situation and the realization that fossil fuels were not only running out but environmentally undesirable, led to a shift in public opinion. The renewed public interest was so strong that it generated an incredible amount of publicity. Most of the major publications printed stories about hydrogen. Articles appeared in Business Week, Readers Digest, Time, Scientific American, and Fortune. Hydrogen had become a popular solution to the prevailing urgency to find a source of domestic energy.
The upsurge in interest led to the formation of advocate groups. The Hindenburg Society, formed on the 35th anniversary of the Hindenburg disaster, was dedicated to the “safe utilization of hydrogen as a fuel.” The purpose was to educate and dispel many of the myths which deemed hydrogen to be a dangerous, useless substance. Originally an informal group, popular interest led to the necessity for the formation of the International Association for Hydrogen Energy in 1974. Another important group, the Institute of Gas Technology, also played an important role in generating public awareness and support for hydrogen research. Other associations that have sprouted up since then include the American Hydrogen Association and the National Hydrogen Association.
Great gains were made in the research of hydrogen in the 1970s, but interest waned in the decade to follow. The reason was once again economics. Hydrogen was still too expensive. Although the environmental aspects were appealing, they could not outweigh the fact that natural gas, oil, and coal were much cheaper and easier to use. Also, the “crisis” in the Middle East dissipated when OPEC loosened its grip, and the price of oil leveled off.
Most advances since the 1970s have been made using hydrogen in motor-driven vehicles, either in conjunction with other fuels, or used in electric vehicles. Since 1982, Georgetown University has been developing a fuel-cell/battery-operated bus. The buses have been used in California, Washington D.C., and Chicago with favorable results. Canada’s Ballard Power Systems developed a 20-passenger bus to run on a hydrogen fuel-cell. Daimler Benz, in Europe has also developed vehicles which run on metal hydride storage systems. A press release dated May 14, 1996, gave details of a newly unveiled fuel cell vehicle available to the public. The NECAR II fuel cell vehicle has room for six people. The fuel cells produce an output of 50 kW and enable a top speed of 110 km/h. The range of the vehicle, on full hydrogen tanks, is more than 250 kilometers. [1]
Daimler-Benz has also mentioned development of an automobile that will produce hydrogen on-board, using methanol. A successful method of producing hydrogen “on the go” would be a major step in hydrogen evolution, and would create a revolution in transportation, even if hydrocarbon fuels are still used.
Other automobile manufacturers, such as Mazda and Renault, have developed hydrogen powered vehicles, although none have been slated for public availability as of yet. Some U.S. companies, pushed by stiffer environmental legislature, and deadlines to produce “zero-emission” automobiles by the year 2000, have increased the push to make available a hydrogen-powered passenger vehicle. There is little evidence that the American automobile manufacturers are able to meet any of the environmental goals set by State and Federal legislatures. Whether this is technical inability or a conflict of interests is unclear.
In the United States, recent legislation has paved the way for hydrogen programs. In 1990, the Spark M. Matsunaga Hydrogen, Research, Development and Demonstration Act (PL 101-566) led to the enactment of a 5-year management and implementation plan for hydrogen research and development. The Hydrogen Technical Advisory Panel was established for coordination and consultation.
The Energy Policy Act of 1992 (PL 102-486) authorized the Department of Energy to administer the five year R&D program. In accordance with the Matsunaga Act, the program would include investigation into renewable production of hydrogen, transportation of hydrogen via existing natural gas pipeline systems, hydrogen storage for vehicle use, and fuel cells for hydrogen powered vehicles.
The Hydrogen Energy Research Program was introduced in the Hydrogen, Fusion and High Energy and Nuclear Physics Research Act of 1994. The bill authorized $134 million over four years. The main goal is the demonstration of the practicability of using hydrogen in transportation, industrial, residential, and utility applications by the year 2000. The bill passed the House but did not pass the Senate. The Hydrogen Future Act of 1995 was a toned down version of the original bill which reduced the emphasis on demonstration projects, and instead focussed more on R&D. The bill passed congress and is now in effect with much funding going into R&D.
Today, interest in hydrogen seems to be on an upswing once again. Recognition of the benefits of hydrogen has reached a global scale. The continued demonstration of the attainability of a renewable, clean-burning fuel has captured public awareness, and has won the support of those governments which aid in funding research and creating infrastructure webs.
Present Technologies
There are four processes which must be considered when developing a hydrogen-fuel system. These processes are:
1. Production
2. Storage
3. Transportation
4. Energy conversion
There are many alternatives from which to choose when developing a hydrogen system. The factors in which each alternative is considered, involve efficiency, economic feasibility, and environmental impacts. How these factors are weighted against each other is open for debate. Currently the prevailing trend is to consider cost-effectiveness above all else. Recent trends in legislature and public concern are shifting emphasis more towards renewable and pollution-free considerations as a priority for development of hydrogen technology.
Hydrogen is a secondary source of energy, not a primary source like oil or natural gas. Therefore, in order to be utilized hydrogen must first be produced. There are many ways in which hydrogen can be produced. Methods of production include chemical, electrochemical, photochemical, biological, and thermochemical processes.
The simplest method to produce hydrogen is to dissolve metals in acid. For example, when zinc (Zn) is placed in a solution of hydrochloric acid, it reacts to produce zinc chloride and hydrogen.
Zn + 2HC1 > ZnC12 + H2
This reaction can be reproduced simply in the laboratory, although the amount of hydrogen produced is minimal. Still, this method was used to a large extent during World War II when scrap aluminum was dissolved in sodium hydroxide (lye) in order to generate hydrogen. The hydrogen was then used to inflate unmanned balloons for weather observation and raising radio antennas. [13] This method is relatively expensive, and is not considereda method for mass production (today, research is being done with scrap iron to produce hydrogen, for use in transportation as a method of producing hydrogen onboard). Small amounts of hydrogen can then be economically produced to provide the needs of a small hydrogen-fuel system.
The cheapest, and by far the most widely used method for producing hydrogen is steam reformation. Steam, and a carbon-based feedstock (usually methane or natural gas), are combined under high temperature and high pressure to produce carbon dioxide and hydrogen. It is estimated that 95% of hydrogen produced in the US is by the steam methane reformation method. [8] Most of this hydrogen is used in industrial applications. Although hydrogen can be produced in this manner for about $0.65 per kilogram, the environmental consequences of the use of hydrocarbons are still a concern. The production of carbon dioxide, a “greenhouse gas,” as well as nitrogen oxides (NOX) contribute to the pollution of the Earth’s atmosphere. Also, the limited resources can only make the cost increase as the supplies of fossil fuel sources decrease. A newly developing renewable option is the use of biomass, or recycled carbonaceous material, as the feedstock in the steam reformation process. The air pollution problems still exist, but it will be an intelligent use of a waste product.
Another method for producing hydrogen is electrolysis. Electrolysis involves the application of a small voltage (approx. 2V DC) to pure water. The electrical energy decomposes the water molecule into its constituent elements, hydrogen and oxygen. This technique has the advantage of producing hydrogen directly from water, with none of the environmental drawbacks which accompany processes using fossil-fuels. Still, the relatively low-efficiency (currently 60-65% with a theoretical maximum of 85%) of the process, and the high cost of electricity make this an expensive option. [10] The cost of producing hydrogen via electrolysis is about $3.00 per kg. [8]
The method of electrolysis is the most attractive for those interested in a completely clean, renewable process using solar energy to produce the electricity. Photovoltaic cells, hydropower, and wind turbines are currently being used to generate the electricity required to electrolyze water for hydrogen production.5, 6 Other renewable options include geothermal, tidal, wave action, and thermal gradients in the ocean. Although most of these processes do not produce sufficient amounts of energy to provide hydrogen on a large scale, on-site electricity production coupled with a small on-site electrolyzer can produce enough energy to provide for the energy needs of a household along with fuel for the family automobile. This allows hydrogen to be produced easily without having to wait for an infrastructure to develop.
Other attempts at water-splitting have involved super-heating water to temperatures high enough to liberate the hydrogen from the water molecule (thermochemical). The temperatures required are in the range of 5000°-6000° F. Adding chemicals such as sulfuric acid can lower the required temperature but the bottom line is that the only feasible way of generating the heat required is by way of a nuclear reaction. Nuclear power generation, needless to say, has severe safety implications. There is still research being done in the thermochemical production of hydrogen which doesn’t require nuclear power plants. An example would be solar power plants in which the heat of the Sun is focused into a tiny point where the heat accumulates, much like a magnifying glass. Yet there are still environmental concerns due to the chemicals involved, and the nitrogen oxides which are formed from a heat reaction in air (which has a high concentration of nitrogen).
Photoprocesses involve the use of light energy for the production of hydrogen. These methods in one way or another, attempt to mimic the natural phenomena of photosynthesis. In plants, chlorophyll captures light energy and uses it to produce complex sugar-phosphate compounds. The most astonishing fact is that this chemical reaction, basically CO2 + H2O + light energy > [51] sugars + O2 occurs at room temperature! Much research has been done to reproduce this feat. Photobiological techniques which coax photosynthetic plants, algae, and bacteria into respiring hydrogen, photochemical techniques which synthetically duplicate the photosynthetic process, and photoelectrochemical techniques which use layers of semiconductors separated by water are being researched today. [4][8] These are promising technologies, but are still in the experimental stage. If efficiency improves, then photoprocesses may play a part in the future of hydrogen.
Storage and Transportation
Hydrogen is typically stored as a liquid, or as a gas. There are advantages and disadvantages to each of these storage options, the choice of which depends upon the ultimate use.
Hydrogen becomes a liquid at temperatures below -423.13° F (-252.9° C). Liquefication of hydrogen is very energy-intensive, with one-third of the energy content of the hydrogen used in the liquefication process.10 This is offset by a reduction of volume requirements for hydrogen storage, with much less storage space required for a liquid than a gas. Less volume needed for storage, makes liquid hydrogen the preferred form of hydrogen used in the Aerospace industry with NASA being one of the largest consumers of liquid hydrogen in the world. [4][12]
Once in liquid form, hydrogen can be transported in pressurized tanks by truck, barge, or rail. Due to the very low boiling temperature of hydrogen, losses due to boil-off can be considerable. Insulation of the tanks is of utmost importance to reduce these losses. If insulated properly, hydrogen can be stored for as much as five years without significant losses. [9][10]
Hydrogen can also be stored as a pressurized gas. As a gas it can be transported via pipelines, using existing natural gas distribution lines. A concern would be possible embrittlement of the lines due to absorption by the metal fittings. Storage of hydrogen as a gas is the most economical method, but due to the necessity for larger tanks, weight and space requirements can be a problem. It is estimated that the mass of a pressure tank is 100 times the mass of the hydrogen stored within it.10 Higher pressure means less volume required, but the walls need to be reinforced to withstand the greater pressure. Although hydrogen is extremely light, the containers necessary to store gaseous hydrogen can be heavy and bulky.
Another method of storing gaseous hydrogen involves metal hydrides. Certain metals such as magnesium, titanium, or iron, have an affinity for hydrogen. Under certain conditions, these metals will absorb gaseous hydrogen, and store it within its molecular structure. When the hydride is heated, the hydrogen is released. Although energy is required to store and to release the hydrogen, this option has proved attractive for use as a storage medium onboard automobiles. The main reason is that it is much less energy-intensive than the liquefication process, although heat energy is required to release the hydrogen. [4] Also, safety and space concerns are reduced when metal hydride storage is used in automobiles.
There are a variety of other methods being developed for hydrogen storage. These include carbon adsorption, glass microspheres, onboard partial oxidation reactors, and recyclable liquid carriers. Some of these options appear promising, but they will still take some time to develop.
Power Conversion
There are two ways of using hydrogen to generate power. One is simple combustion. The use of hydrogen in internal combustion engines has been used extensively. The other is the conversion of hydrogen into electricity in a fuel cell, which is essentially electrolysis in reverse. Both of these have their advantages and disadvantages.
Internal combustion engines can be easily converted to run on hydrogen, or a hydrogen-fuel mixture. [7][10] The noxious emissions are greatly reduced, with water being the only by-product if pure hydrogen and oxygen are used. Nitrogen oxides are still formed from the high heat of combustion, and are still a source of air pollution.
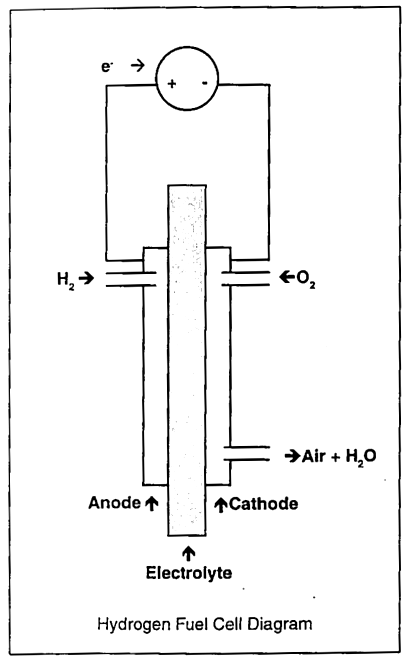
Over the past two decades, most research has gone into the development of the fuel cell. The operation of a fuel cell involves the combination of hydrogen (anode) and oxygen (cathode) in the presence of an electrolyte. Output voltages range from 0.7 to 1.12 V.10 The type of fuel cell varies depending on the electrolyte used. Fuel cell types include the Phosphoric acid fuel cell, the alkaline fuel cell, and the solid oxide fuel cell. The most common type, the alkaline fuel cell, is still used by NASA on board spacecraft.7 Another type of electrolyte being developed is the proton-exchange membrane which uses a solid polymer to facilitate the reverse electrolysis process. This solid polymer, which is much like plastic kitchen wrap, conducts protons, and is very conducive to the purpose of an electrolyte. Although membrane costs are high, this type of fuel cell appears very promising, and is currently being used in advanced research (Schatz Lab, Humboldt State University, California).
The use of hydrogen is at an all-time high. It is possible to convert any car sitting in the driveway to run on hydrogen. It is being proven every day that hydrogen can be used as a replacement not only for gasoline, but natural gas in heaters and stoves in the home. Hydrogen could some day replace electricity as the primary energy-carrier via high-voltage power lines, being transported in pipelines and converted to electricity on-site.
Production of hydrogen is also becoming easy to do for anyone with access to about 2V of DC electricity. Many homesteads generate enough electricity using windmills and solar panels to supply the household’s needs. A small electrolyzer added to this system could easily produce enough hydrogen to fuel a vehicle. It is clearly possible that anyone with a little ingenuity and skill can convert the household to use hydrogen, convert the car to run on hydrogen, and generate the electricity for hydrogen production using only solar energy, all for about the cost of a mid-sized American sedan.
Any in depth study of hydrogen reveals the vast array of system configurations for hydrogen power. The bottom line is that any system which utilizes hydrogen in any capacity is going to be better off for it. Harmful emissions are reduced, efficiency is increased and water(the original source), is produced. On a larger level, it would seem possible that use of hydrogen alone or in conjunction with other fuels would be a major step in the right direction, and bring us a little closer to a more harmonic cycle of energy use.
References
- Daimler-Benz A.G., “News Release: Fuel Cell Vehicle NECAR II”, National Hydrogen Association Web Page (http://www.paltech.com/ttc/NHA/), 1996.
- David Halliday, Robert Resnick, and John Merrill, Fundamentals of Physics, John Wiley & Sons, 1988. <>
- Gladys Hefferlin, and W. C. Hefferlin, Hefferlin Manuscripts: Part I & II, BSRF, op.
- Peter Hoffmann, The Forever Fuel: The Story of Hydrogen, Westview Press, 1981. <>
- Peter Lehman, and Christine Parra, “Hydrogen Fuel From the Sun,” Solar Today, Sept.-Oct. 1994.
- Peter Lehman, and Charles E. Chamberlin, “Design and Performance of SERC’s Prototype Fuel Cell Powered Vehicle”, 7th Annual National Hydrogen Association Meeting, April 2-4, 1996.
- James MacKenzie, The Keys to the Car: Electric and Hydrogen Vehicles for the 21st Century, World Resources Institute, 1994. <>
- Daniel Morgan, and Fred Sissine, “Hydrogen: Technology and Policy”, Congressional Research Service Report for Congress, Committee for the National Institute for the Environment, 1995.
- Joan M. Ogden and Robert H. Williams, Solar Hydrogen: Moving Beyond Fossil Fuels, World Resources Institute, 1989. <>
- Michael A. Peavey, Fuel From Water: Energy Independence With Hydrogen, Merit Products, Inc., 1983. <>
- William K. Purves, Gordon H. Orians, and H. Craig Heller, Life: The Science of Biology, Sinauer Assoc., inc., 1992. <>
- Luther W. Skelton, The Solar-Hydrogen Energy Economy: Beyond the Age of Fire, Van Nostrand Reinhold Co., 1984. <>
- L.O. Williams, Hydrogen Power: An Introduction to Hydrogen Energy and its Applications, Permagon Press, 1980. <>
- Steven S. Zumdahl, Chemistry, D. C. Heath and Co., 1986. <>